The post-operative rehabilitation, to protect the newly formed tissue during the initial remodelling phase, must commence immediately after the operation.
The Actifit rehab booklet provides a guideline for a rehabiliation program.
Please download our treatment algorithm for a guideline on patient selection.


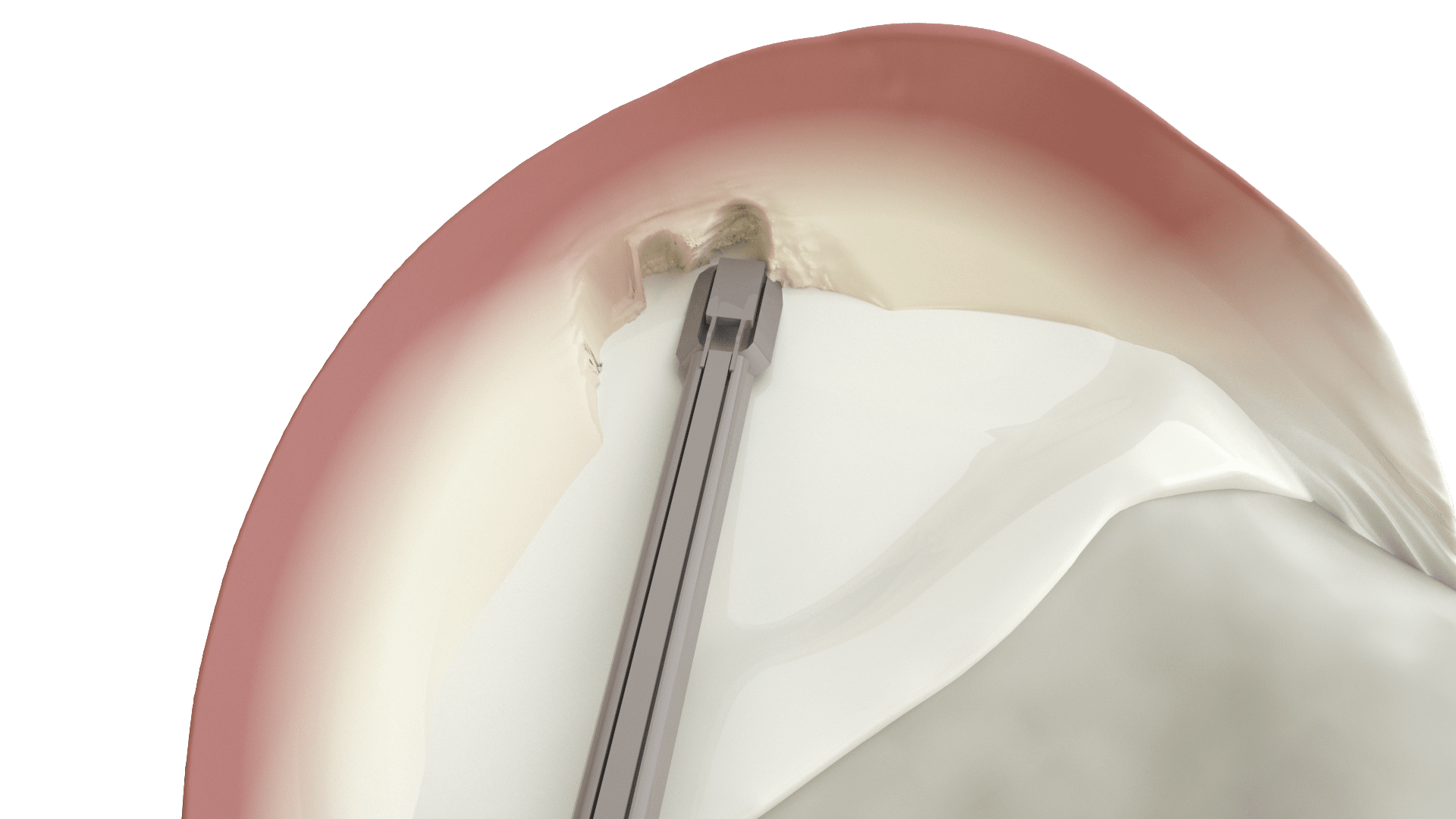
Scaffold facilitates tissue regeneration from the vascularized portion of the meniscus

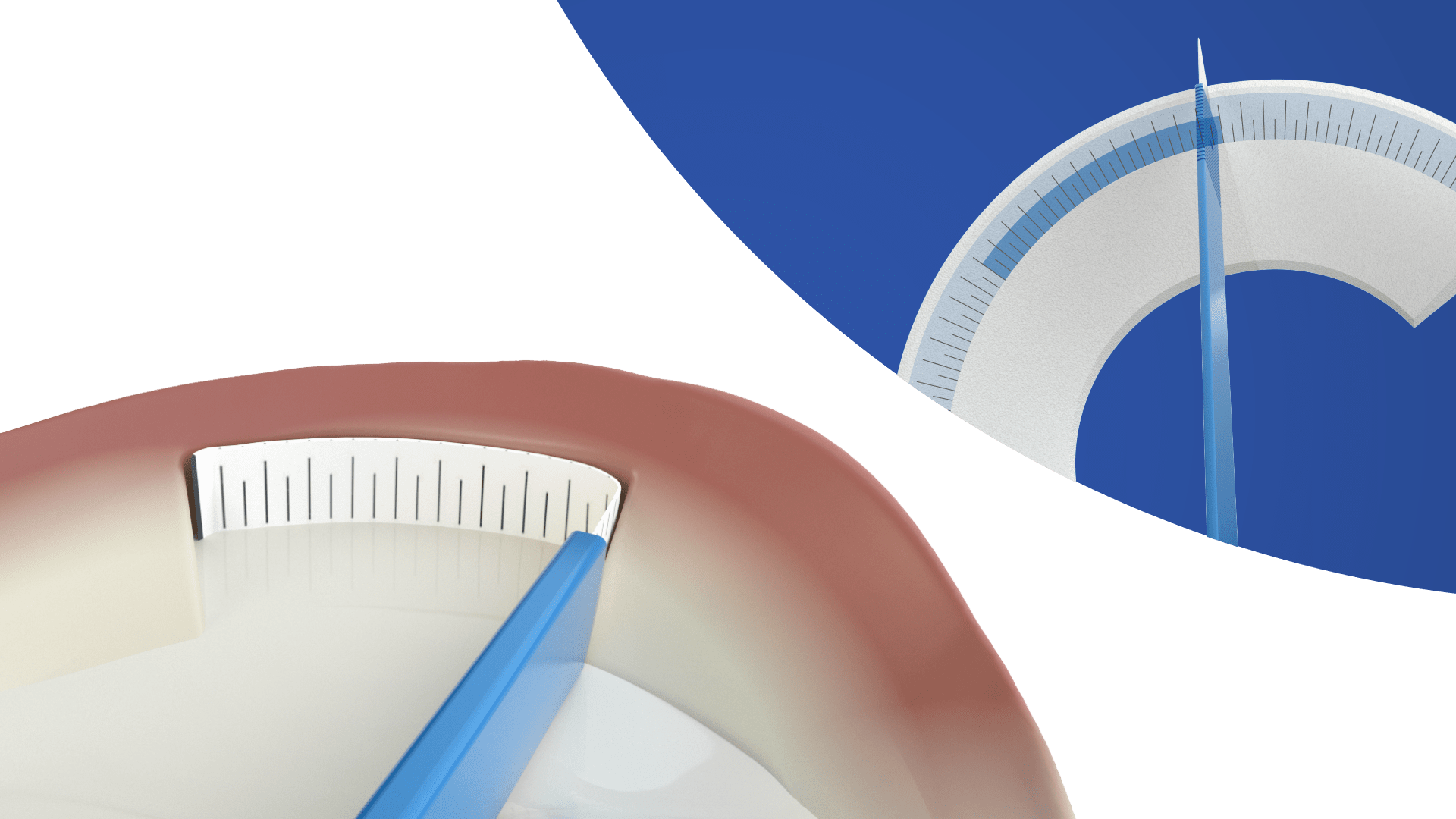
Shaped and sized for medial or lateral meniscus defects

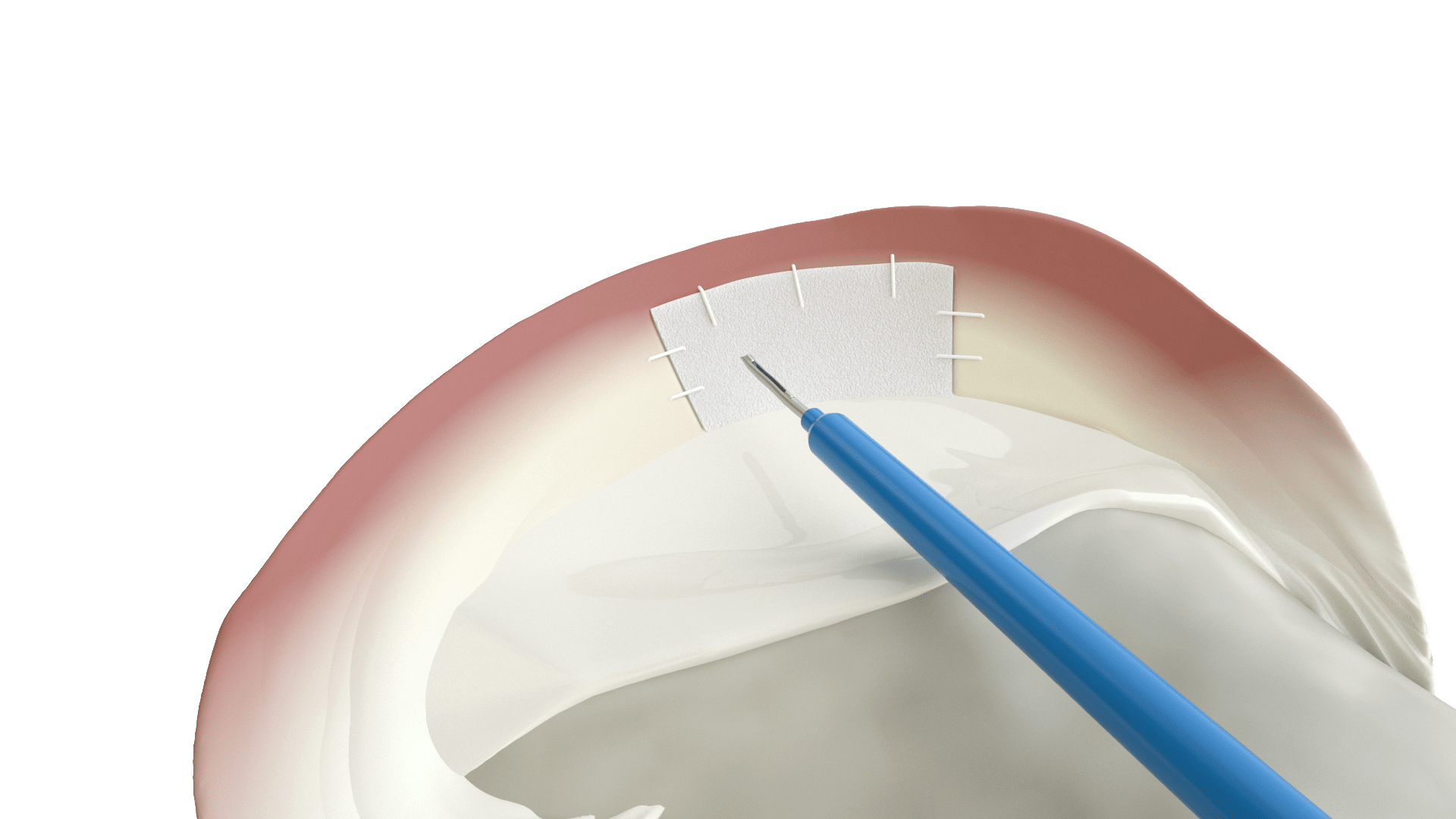
Reliable handling and suture fixation.

Slow degradation. Facilitates tissue remodelling
The results speak for themselves.
KOOS Sports
KOOS QoL
VAS Pain
IKDC
Toanen et al. (2020). Polyurethane Meniscal Scaffold for the Treatment of Partial Meniscal Deficiency: 5-Year Follow-up Outcomes: A European Multicentric Study.
View publicationActifit has significant potential to improve joint health and delay the need for joint replacement. Actifit is CE marked. Actifit is not currently available in the United States.
Our products have been validated in over
30 peer-reviewed clinical publications and cited in over 100 journal articles.
Every year there are more than 1,500,000 meniscectomies and 630,000 total knee replacements performed in the US and Europe. Partial meniscectomy outcomes can be drastically improved by using Actifit to fill the defect, especially if you are experiencing knee symptoms following a prior meniscus surgery. Our goals are to delay or prevent total knee replacement by saving the meniscus.
At Orteq, we have created a unique joint preservation solution for orthopaedic/sports medicine surgeons that encourage a patient’s own natural healing process. Our Actifit solution offers an innovative regenerative medicine approach to soft tissue defects in joints for patients suffering from injury or degeneration
W